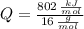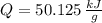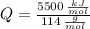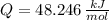Cuando se quema 1 mol de metano –o sea, 16 g–, se desprenden 802
kJ/mol.
○ Cuando se quema 1 mol de octano –o sea, 114 g–, se desprenden 5500
kJ/mol.
Pareciera que el octano puede brindar más energía al quemarse, pero vamos a
hacer un análisis más cuidadoso. Comparemos la combustión de igual masa de
cada combustible.
● Calculen la cantidad de calor que se desprende cuando se quema un gramo
de cada combustible.
1 gr de metano aporta……………………………
1 gr de octano aporta………………………………

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Cuando se quema 1 mol de metano –o sea, 16 g–, se desprenden 802
kJ/mol.
○ Cuando se quema 1...
○ Cuando se quema 1...
Questions

Mathematics, 02.09.2020 19:01


History, 02.09.2020 19:01

Mathematics, 02.09.2020 19:01



Mathematics, 02.09.2020 19:01

History, 02.09.2020 19:01

Computers and Technology, 02.09.2020 19:01




Mathematics, 02.09.2020 19:01

Mathematics, 02.09.2020 19:01


Geography, 02.09.2020 19:01

English, 02.09.2020 19:01



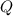 ), en kilojoules por mol, es igual a la cantidad de energía liberada por mol de compuesto (
), en kilojoules por mol, es igual a la cantidad de energía liberada por mol de compuesto (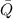 ), en kilojoules por mol, dividido por su masa molar (
), en kilojoules por mol, dividido por su masa molar (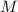 ), en gramos por mol:
), en gramos por mol: (1)
(1)