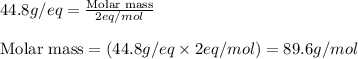Chemistry, 30.06.2021 05:50 CelesteN64
Calculate the molecular weight of a dibasic acid.0.56gm of which is required 250ml of N/20 sodium hydroxide solution for neutralization.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Calculate the molecular weight of a dibasic acid.0.56gm of which is required 250ml of N/20 sodium hy...
Questions

Biology, 04.06.2021 19:30

Biology, 04.06.2021 19:30




Mathematics, 04.06.2021 19:30

Mathematics, 04.06.2021 19:30




Mathematics, 04.06.2021 19:30

Mathematics, 04.06.2021 19:30



Engineering, 04.06.2021 19:30

Mathematics, 04.06.2021 19:30



Mathematics, 04.06.2021 19:30

Mathematics, 04.06.2021 19:30

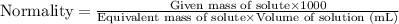 ....(1)
....(1)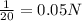
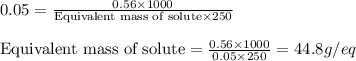
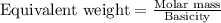 .....(2)
.....(2)