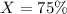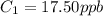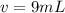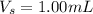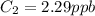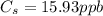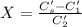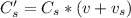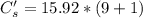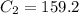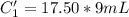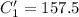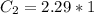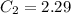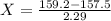A sample of drinking water was tested for Pb2 and was found to have a Pb2 concentration of 17.50 ppb. A 9.00 mL sample of the drinking water was spiked with 1.00 mL of a 2.29 ppb Pb2 standard. Analysis of the spiked sample gave a concentration of 15.93 ppb Pb2 . Find the percent recovery of the spike.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2


Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
A sample of drinking water was tested for Pb2 and was found to have a Pb2 concentration of 17.50 ppb...
Questions







Mathematics, 13.07.2019 22:00





Social Studies, 13.07.2019 22:00

History, 13.07.2019 22:00




Mathematics, 13.07.2019 22:00

Mathematics, 13.07.2019 22:00

Computers and Technology, 13.07.2019 22:10

Computers and Technology, 13.07.2019 22:10