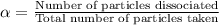Chemistry, 21.06.2021 14:00 atsuedem974
Zinc sulfate is a 2-ion electrolyte,
dissociating 40% in a
certain concentration. Calculate its
dissociation (i) factor.
On the basis of 40% dissociation, 100
particles of zinc sulfate
will yield:
40zinc ions
40 sulfate ions
60undissociated particles
Jo 11:03

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1


Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1
You know the right answer?
Zinc sulfate is a 2-ion electrolyte,
dissociating 40% in a
certain concentration. Calculate i...
certain concentration. Calculate i...
Questions




Mathematics, 19.03.2020 00:30

Mathematics, 19.03.2020 00:30




English, 19.03.2020 00:30

Mathematics, 19.03.2020 00:31


Biology, 19.03.2020 00:31



Mathematics, 19.03.2020 00:31

Mathematics, 19.03.2020 00:31


Mathematics, 19.03.2020 00:31


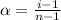
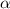 = degree of dissociation = 40% = 0.40
= degree of dissociation = 40% = 0.40