Chemistry, 19.03.2020 00:31 Kjcampbell2
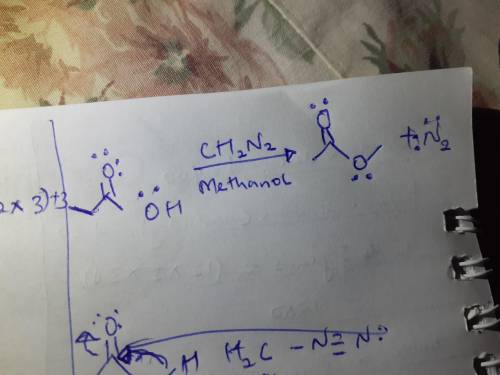
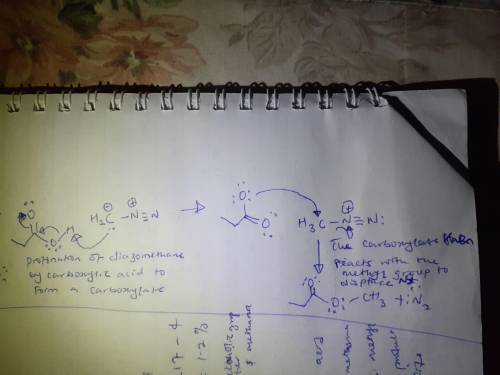
Diazomethane, CH2N2, is used in the organic chemistry laboratory despite its danger because it produces very high yields and is selective for reaction with carboxylic acids. Draw the product of the reaction of this compound with excess diazomethane in methanol. As an aid, the structure of initial compound is provided for you in the drawing window.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1

You know the right answer?
Diazomethane, CH2N2, is used in the organic chemistry laboratory despite its danger because it produ...
Questions

History, 29.11.2020 08:30


Mathematics, 29.11.2020 08:30


Chemistry, 29.11.2020 08:30

Arts, 29.11.2020 08:30

World Languages, 29.11.2020 08:30


Health, 29.11.2020 08:30

Biology, 29.11.2020 08:30

English, 29.11.2020 08:30






Chemistry, 29.11.2020 08:30