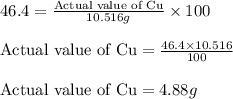Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Aluminum reacts with excess copper(II) sulfate according to the unbalanced reaction
Al(s) + CuSO4(a...
Questions



Chemistry, 24.10.2021 01:00







Geography, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Chemistry, 24.10.2021 01:00

History, 24.10.2021 01:00

Business, 24.10.2021 01:00

Business, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Social Studies, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

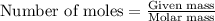 ......(1)
......(1)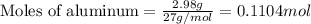
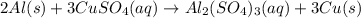
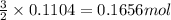 of Cu
of Cu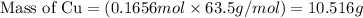
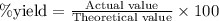 ......(2)
......(2)