
Chemistry, 03.06.2021 05:40 jaygamer37
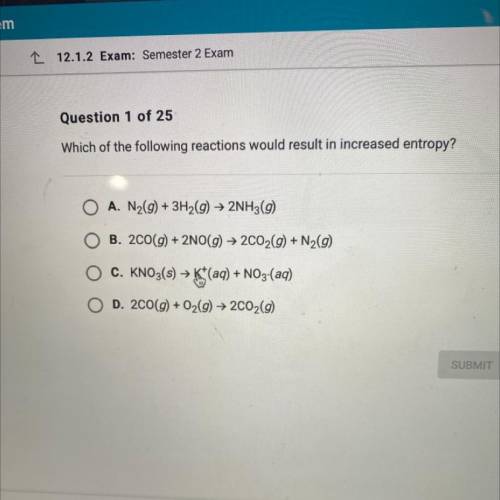
Which of the following reactions would result in increased entropy?
A. N2(g) + 3H2(g) + 2NH3(9)
B. 200(g) + 2NO(g) → 2C02(g) + N2(9)
C. KNO3(s) > K+(aq) + NO3-(aq)
D. 200(g) + O2(g) → 2C02(9)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

You know the right answer?
Which of the following reactions would result in increased entropy?
A. N2(g) + 3H2(g) + 2NH3(9)
Questions


Computers and Technology, 07.10.2019 19:10




Computers and Technology, 07.10.2019 19:10









English, 07.10.2019 19:10







