
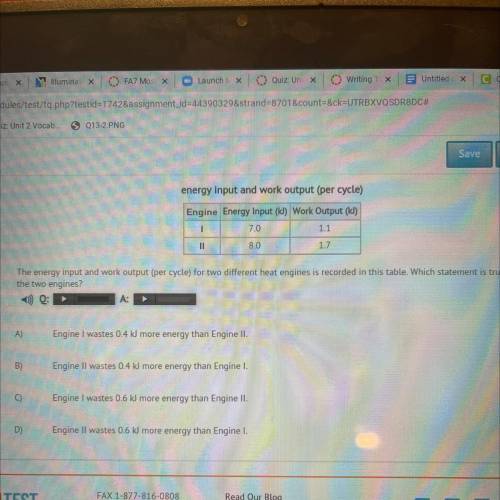
The energy input and work output (per cycle) for two different heat engines is recorded in this table. Which statement is true about
the two engines?
A)
Engine I wastes 0.4 kJ more energy than Engine II.
B)
Engine Il wastes 0.4 kJ more energy than Engine I.
Engine I wastes 0.6 kJ more energy than Engine II.
D)
Engine II wastes 0.6 kJ more energy than Engine I.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
You know the right answer?
The energy input and work output (per cycle) for two different heat engines is recorded in this tabl...
Questions

Mathematics, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01

History, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01

History, 30.09.2020 01:01

Biology, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01

Mathematics, 30.09.2020 01:01


Biology, 30.09.2020 01:01

Computers and Technology, 30.09.2020 01:01



Mathematics, 30.09.2020 01:01


Social Studies, 30.09.2020 01:01


Mathematics, 30.09.2020 01:01




