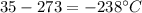Chemistry, 21.05.2021 23:30 superpuglover5924
Pure oxygen boils at -183 ℃ and freezes at -219 ℃. What state will the oxygen be in if the temperature is brought to within 35 K of absolute zero?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Pure oxygen boils at -183 ℃ and freezes at -219 ℃. What state will the oxygen be in if the temperatu...
Questions

Mathematics, 24.02.2021 03:30


Mathematics, 24.02.2021 03:30



Mathematics, 24.02.2021 03:30



Social Studies, 24.02.2021 03:30

Mathematics, 24.02.2021 03:30


Mathematics, 24.02.2021 03:30

Mathematics, 24.02.2021 03:30


Mathematics, 24.02.2021 03:30

Physics, 24.02.2021 03:30


Mathematics, 24.02.2021 03:30