
Chemistry, 19.05.2021 23:40 starlightmoon213
When the temperature of a closed vessel containing a gas is increased by 273 °C, the pressure was found to increase by 20%. What is the initial temperature of the closed vessel?

No spamming or Irrelevant Answer.
Ty!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

You know the right answer?
When the temperature of a closed vessel containing a gas is increased by 273 °C, the pressure was fo...
Questions

Mathematics, 27.10.2020 17:20


History, 27.10.2020 17:20



History, 27.10.2020 17:20




Biology, 27.10.2020 17:20


English, 27.10.2020 17:20





Mathematics, 27.10.2020 17:20


Mathematics, 27.10.2020 17:20

Biology, 27.10.2020 17:20



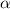 T
T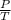 =K where k is constant
=K where k is constant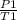 =
=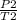
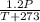
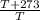 =
=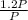
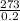 =1365°C or 1365K
=1365°C or 1365K

