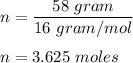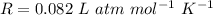Chemistry, 14.05.2021 06:00 tytenaedwards6441
a sample of methane gas (ch4) with a mass of 58 g is kept in a 1.5 L container at a temperature of 373 K. what is the pressure of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
a sample of methane gas (ch4) with a mass of 58 g is kept in a 1.5 L container at a temperature of 3...
Questions


Spanish, 05.05.2021 02:10

Mathematics, 05.05.2021 02:10

Advanced Placement (AP), 05.05.2021 02:10

Mathematics, 05.05.2021 02:10





Social Studies, 05.05.2021 02:10

Mathematics, 05.05.2021 02:10



Mathematics, 05.05.2021 02:10

Mathematics, 05.05.2021 02:10

Mathematics, 05.05.2021 02:10


Spanish, 05.05.2021 02:10