
Chemistry, 13.05.2021 22:20 angieplasencia8
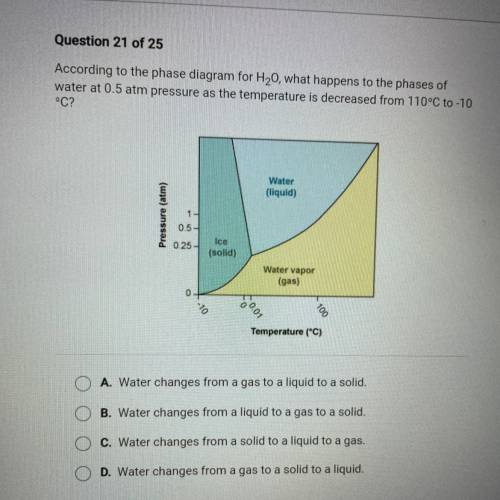
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as the temperature is decreased from 110°C to -10
°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

You know the right answer?
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as...
Questions

Mathematics, 03.11.2020 01:00





Mathematics, 03.11.2020 01:00





Mathematics, 03.11.2020 01:00



English, 03.11.2020 01:00




Mathematics, 03.11.2020 01:00

Social Studies, 03.11.2020 01:00

Health, 03.11.2020 01:00



