
Chemistry, 11.05.2021 08:00 87haymaker
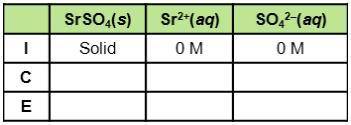
Consider the dissociation of SrSO4, which has a Ksp of 3.2 x 10-7. What do the three rows of (I, C,E) stand for in the table? How can the table be used to find equilibrium constants for this example?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Consider the dissociation of SrSO4, which has a Ksp of 3.2 x 10-7. What do the three rows of (I, C,E...
Questions

Chemistry, 13.01.2020 18:31


Social Studies, 13.01.2020 18:31


Mathematics, 13.01.2020 18:31



Social Studies, 13.01.2020 18:31




English, 13.01.2020 18:31

English, 13.01.2020 18:31





English, 13.01.2020 18:31


History, 13.01.2020 18:31



