Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
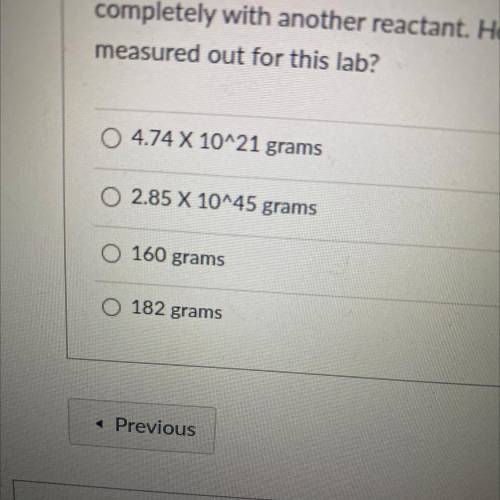
In a stoichiometry lab, 7.21 X 1023 molecules of vanillin, C3H2O3, are needed to react
completely...
Questions


Chemistry, 08.02.2021 19:20





Mathematics, 08.02.2021 19:20


English, 08.02.2021 19:20





Computers and Technology, 08.02.2021 19:20





History, 08.02.2021 19:20

History, 08.02.2021 19:20