Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g)...

Chemistry, 27.04.2021 02:10 coolgirl5679
Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (I), ΔH = -890 kJ/mol
Calculate much heat is released when 3.5 moles of propane have a combustion reaction.
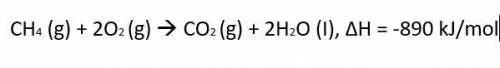

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
You know the right answer?
Questions

English, 07.11.2019 16:31

Advanced Placement (AP), 07.11.2019 16:31


Social Studies, 07.11.2019 16:31


Physics, 07.11.2019 16:31




Chemistry, 07.11.2019 16:31


Biology, 07.11.2019 16:31

Mathematics, 07.11.2019 16:31

Mathematics, 07.11.2019 16:31


Business, 07.11.2019 16:31


Biology, 07.11.2019 16:31

Mathematics, 07.11.2019 16:31



