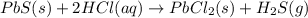Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Solid lead(ii) sulfide reacts with aqueous hydrochloric acid to form solid lead(ii) chloride and dih...
Questions





Mathematics, 20.04.2020 22:31



Social Studies, 20.04.2020 22:31

Mathematics, 20.04.2020 22:31

English, 20.04.2020 22:31

Mathematics, 20.04.2020 22:31


Biology, 20.04.2020 22:31





Mathematics, 20.04.2020 22:31