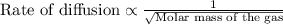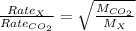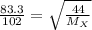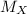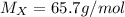Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Agas of unknown identity diffuses at a rate of 83.3 ml/s in a diffusion apparatus in which carbon di...
Questions

Spanish, 15.01.2021 16:50


Biology, 15.01.2021 16:50


Mathematics, 15.01.2021 16:50

Computers and Technology, 15.01.2021 16:50



English, 15.01.2021 16:50


Spanish, 15.01.2021 16:50

Mathematics, 15.01.2021 16:50



Mathematics, 15.01.2021 16:50


Chemistry, 15.01.2021 16:50