ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Gra...

Chemistry, 16.04.2021 20:20 fatimahellis33
ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Graph experimental data and interpret results for peer review
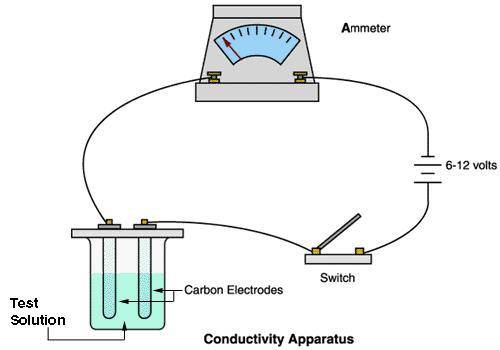
A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This ammeter measures in milliamperes (mA). The following data was taken.
Solution Reading
0.1 M H2SO4 150 mA
0.1 M Ba(OH)2 150 mA
To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The following results were recorded.
DATA TABLE
Total H2SO4 Added Meter Reading Observations
0 mL 150 mA Ba(OH)2 and H2SO4 clear, colorless
10 mL 65 mA milky white precipitate forms
20 mL 31 mA more precipitate forms
30 mL 0 mA precipitate heavy and settles
40 mL 29 mA no added precipitate seen to form
50 mL 62 mA no change seen
Questions
1. Did you plot the data?
yes
no
2. Did you label your graph axes?
no
yes
3. Did you give your graph a title?
no
yes
4. Does the Ba(OH)2 solution contain ions?
yes
no
5. Does the H2SO4 solution contain ions?
yes
no
6. Explain the data.
Is there any evidence that a reaction has occurred?
7. Does the conductivity increase or decrease?
8. Does the number of ions in solution increase, decrease, or remain constant?
9. What is the indicator of the number of ions in solution?
10. How does this evidence indicate that the reaction has occurred between ions?
11. The Ba(OH)2 dissociates as Ba+2 + 2 OH-. H2SO4 dissociates as 2 H+ + SO4-2.
Write a balanced equation for this reaction.
12. When the conductivity is at a minimum, what must be true about the amount of Ba(OH)2?
13. Why does it not conduct at this low point?
14. Why does it conduct more before and after this minimum point?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:30
98 points you will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. before you conduct your experiment, you need to form a hypothesis. a hypothesis is a prediction of what you think will happen in the experiment. the hypothesis is a statement that describes “if” a certain set of circumstances are present “then” there will be a specific result that will occur. record your hypothesis here: record the results from step one of the experiment (dropping the objects in the air): first trial: second trial: third trial: record the results from step two of the experiment (dropping the objects in a vacuum): first trial: second trial: third trial: did the experiment support your hypothesis? using the data from your experiment, describe why you believe your hypothesis was either proven or disproven. what forces were acting on the objects dropped in the air? what force was acting on the objects dropped in the vacuum? part two: comparing forces choose two forces and compare and contrast these forces. you must provide two ways that they are alike and two ways that they are different. you may make a list, write in paragraph form, or make a chart. choose two forces and compare and contrast these forces. these must be different forces than used in the prior question. provide two ways that they are similar and two ways that they are different. you may make a list, write it out, or make a chart.
Answers: 3

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Questions


Biology, 14.10.2019 07:30



English, 14.10.2019 07:30

Biology, 14.10.2019 07:30


English, 14.10.2019 07:30






Mathematics, 14.10.2019 07:30





History, 14.10.2019 07:30

Business, 14.10.2019 07:30



