
Chemistry, 16.04.2021 14:00 valeriegarcia12
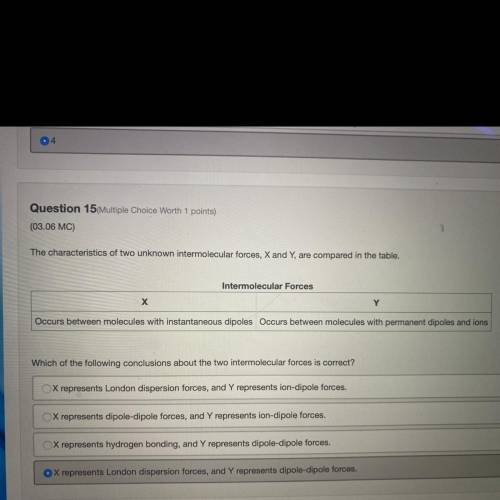
The characteristics of two unknown intermolecular forces, X and Y, are compared in the table.
Intermolecular Forces
х
Y
Occurs between molecules with instantaneous dipoles Occurs between molecules with permanent dipoles and ions
Which of the following conclusions about the two intermolecular forces is correct?
X represents London dispersion forces, and Y represents ion-dipole forces.
X represents dipole-dipole forces, and Y represents ion-dipole forces.
X represents hydrogen bonding, and Y represents dipole-dipole forces.
X represents London dispersion forces, and Y represents dipole-dipole forces.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
The characteristics of two unknown intermolecular forces, X and Y, are compared in the table.
Inter...
Questions




Physics, 16.06.2021 21:40

Mathematics, 16.06.2021 21:40










Mathematics, 16.06.2021 21:40



Physics, 16.06.2021 21:40

Mathematics, 16.06.2021 21:40

Mathematics, 16.06.2021 21:40



