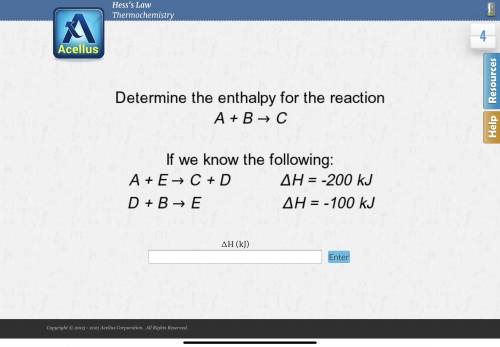
Determine the enthalpy for the reaction A + B -> C
If we know the following:
A + E ->...

Chemistry, 08.04.2021 22:20 Lilbre6999
Determine the enthalpy for the reaction A + B -> C
If we know the following:
A + E -> C + D deltaH = -200 kJ
D + B -> E deltaH = -100 kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Questions





Chemistry, 04.06.2021 20:10







Mathematics, 04.06.2021 20:10



Chemistry, 04.06.2021 20:10

Mathematics, 04.06.2021 20:10

Mathematics, 04.06.2021 20:10

Mathematics, 04.06.2021 20:10

Mathematics, 04.06.2021 20:10




