Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
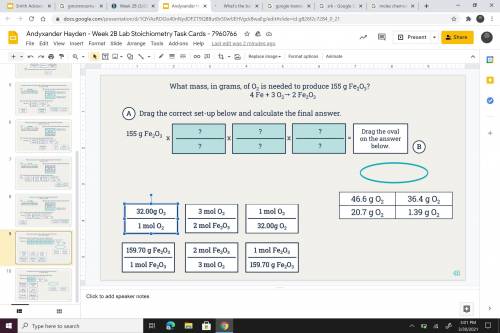
What mass, in grams of O2 is needed to produce 155 g Fe2 O3? 4 Fe + 3 O2---> 4 Fe + 3 o2 --> 2...
Questions

Mathematics, 13.11.2019 07:31




Mathematics, 13.11.2019 07:31


Biology, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31

History, 13.11.2019 07:31



Mathematics, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31


English, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31

English, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31