
Help please || will mark brainlest || ten pts <3
Which is true about the reaction shown above?
Group of answer choices
The reaction is a single replacement.
The reaction releases energy.
The reaction has two elements that combine to form a compound.
The reaction has negative ions in two of the compounds that switch places.
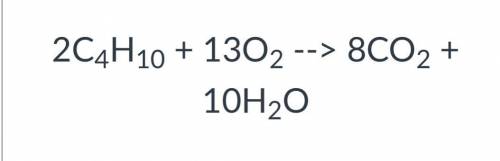

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
Help please || will mark brainlest || ten pts <3
Which is true about the reaction shown above?
Questions







Mathematics, 15.07.2019 17:30

Mathematics, 15.07.2019 17:30




Physics, 15.07.2019 17:30

History, 15.07.2019 17:30




English, 15.07.2019 17:30

Mathematics, 15.07.2019 17:30

Biology, 15.07.2019 17:30



