
Chemistry, 18.03.2021 02:00 boogerbuttday
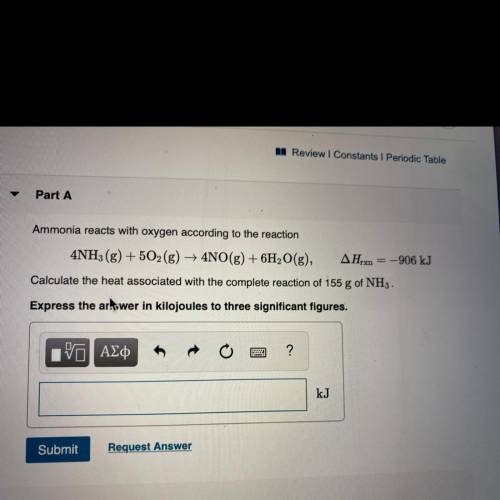
Ammonia reacts with oxygen according to the reaction
4NH3(g) + 5O2(g) + 4NO(g) + 6H2O(g), AHrx = -906 kJ
Calculate the heat associated with the complete reaction of 155 g of NH3 .
Express the artswer in kilojoules to three significant figures.
Se
ΑΣΦ
?
kJ
her
9.pdf
Submit
Request Answer
Part B

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

You know the right answer?
Ammonia reacts with oxygen according to the reaction
4NH3(g) + 5O2(g) + 4NO(g) + 6H2O(g), AHrx = -9...
Questions




Mathematics, 24.09.2019 12:00

Mathematics, 24.09.2019 12:00

Mathematics, 24.09.2019 12:00


Health, 24.09.2019 12:00

Mathematics, 24.09.2019 12:00



Mathematics, 24.09.2019 12:00

History, 24.09.2019 12:00









