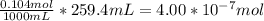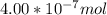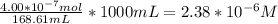Chemistry, 24.09.2019 12:00 divadebbgirl1
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sodium hydroxide (naoh) , she finds that it requires 259.4 ml of the base to reach the endpoint of the titration. what is the molarity of the acid solution ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

You know the right answer?
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sod...
Questions

Mathematics, 14.02.2020 00:29








Chemistry, 14.02.2020 00:29


Mathematics, 14.02.2020 00:29



Mathematics, 14.02.2020 00:29