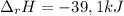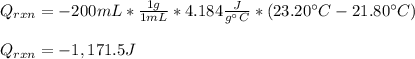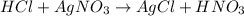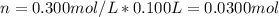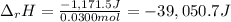100 mL of a 0.300 M solution of AgNO3 reacts with 100 mL of a 0.300 M solution of HCl in a
coffee-cup calorimeter and the temperature rises from 21.80 °C to 23.20 °C. Assuming the density
and specific heat of the resulting solution is 1.00 g/mL and 4.18 J/g. °C, respectfully, what is the
AHºx?
A 39.0 kJ/mol
B +39.0 kJ/mol
C.+1.17 kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

You know the right answer?
100 mL of a 0.300 M solution of AgNO3 reacts with 100 mL of a 0.300 M solution of HCl in a
coffee-c...
Questions

Mathematics, 22.02.2021 23:40

History, 22.02.2021 23:40

History, 22.02.2021 23:40

Mathematics, 22.02.2021 23:40


Mathematics, 22.02.2021 23:40










Mathematics, 22.02.2021 23:40