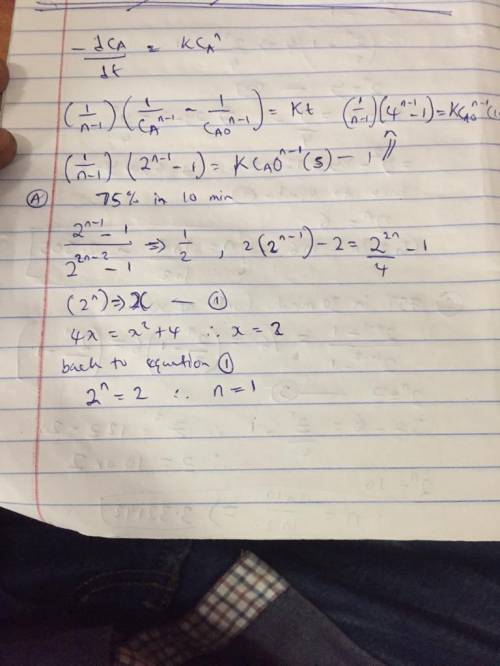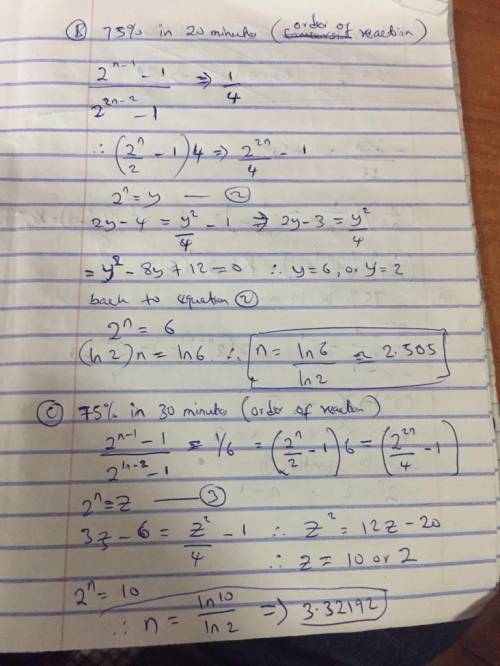Chemistry, 28.02.2021 17:00 adjjones2011
Liquid A decomposes with nth-order kinetics in a batch reactor A->B, r =kca. the conversion of A reaches 50% in a five minute run.
a. What is the order of the reaction if it takes 10 minutes to reach 75% conversion?
b. What is the order of the reaction if it takes 20 minutes to reach 75% conversion?
c. What is the order of the reaction if it takes 30 minutes to reach 75% conversion?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

You know the right answer?
Liquid A decomposes with nth-order kinetics in a batch reactor A->B, r =kca. the conversion of A...
Questions

English, 07.01.2021 17:30

Mathematics, 07.01.2021 17:30


Mathematics, 07.01.2021 17:30






Chemistry, 07.01.2021 17:30



History, 07.01.2021 17:30