
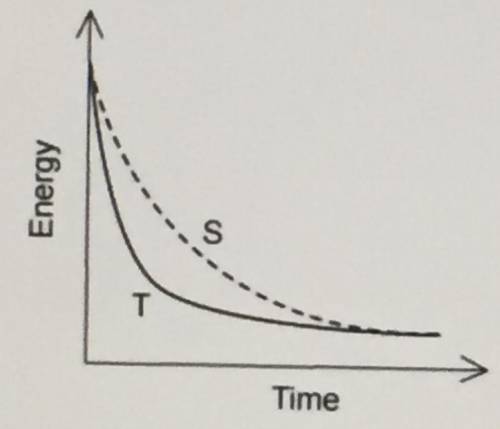
A student was investigating the rate of reaction between a solid base and a solution of
sulfuric acid. Two experiments were performed, S and T, in which the mass of the
reaction flask was recorded as shown in the graph.
Which of the following changes could explain the difference in results between S and T?
A ) The sulfuric acid is less concentrated in T.
B ) The sulfuric acid is more concentrated in T.
C ) A higher temperature is used in S.
D) Larger sized particles are used in T.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
What does standard deviation reveal about data? a. the average of all the data points b. which of the data points is most reliable c. how spread out the data points are d. the percent error included in the data
Answers: 2

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3
You know the right answer?
A student was investigating the rate of reaction between a solid base and a solution of
sulfuric ac...
Questions

Mathematics, 09.02.2021 04:50


Mathematics, 09.02.2021 04:50

Mathematics, 09.02.2021 04:50

Chemistry, 09.02.2021 04:50




Mathematics, 09.02.2021 04:50

Mathematics, 09.02.2021 04:50


Health, 09.02.2021 04:50




Mathematics, 09.02.2021 04:50



History, 09.02.2021 04:50

Mathematics, 09.02.2021 04:50



