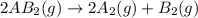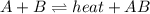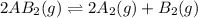1. chemical equilibrium is established when the number of reactants equals the number of products.. - true. - false. 2. according to le chatelier's principle, by increasing the temperature of the system shown below, the equilibrium will shift to the right (towards . a + b heat + ab. - true. - false. 3. for which of the following reactions will an increase in pressure not effect the position of equilibrium? . . a. 2a2 (g) + b2 (g) 2a2b (g). b. 2ab (g) a2 (g) + b2 (g). c. 2a (g) + f2 (g) 2af (g). d. 2b (s) + 2ha (aq) 2ba (aq) + h2 (g).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
1. chemical equilibrium is established when the number of reactants equals the number of products.....
Questions

Mathematics, 21.06.2021 01:00

Mathematics, 21.06.2021 01:00

Mathematics, 21.06.2021 01:00


Mathematics, 21.06.2021 01:00

Mathematics, 21.06.2021 01:00


Mathematics, 21.06.2021 01:00

English, 21.06.2021 01:00



World Languages, 21.06.2021 01:00

Mathematics, 21.06.2021 01:00



Mathematics, 21.06.2021 01:00


History, 21.06.2021 01:00

Business, 21.06.2021 01:00

English, 21.06.2021 01:00