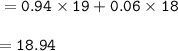Chemistry, 06.12.2020 16:50 davienwatson8
A sample of fluorine contains isotopes with different masses; F-19 and F-18. In the sample 94% of the atoms are F-19. Calculate the relative mass of the sample.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
A sample of fluorine contains isotopes with different masses; F-19 and F-18. In the sample 94% of th...
Questions


Computers and Technology, 07.03.2020 02:23












Mathematics, 07.03.2020 02:23