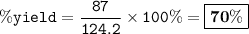Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
W...

Chemistry, 26.11.2020 01:00 pinkpearl20
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
When David reacts 13.8 grams of hydrogen gas with excess oxygen, 87.0 grams of water are formed. Calculate his percent yield of water.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
Questions

English, 08.09.2020 17:01

English, 08.09.2020 17:01






English, 08.09.2020 17:01

Mathematics, 08.09.2020 17:01


Social Studies, 08.09.2020 17:01

Computers and Technology, 08.09.2020 17:01

Arts, 08.09.2020 17:01

English, 08.09.2020 17:01

Biology, 08.09.2020 17:01

Biology, 08.09.2020 17:01

Spanish, 08.09.2020 17:01

Mathematics, 08.09.2020 17:01

Mathematics, 08.09.2020 17:01

Mathematics, 08.09.2020 17:01