
Chemistry, 24.11.2020 14:00 nagwaelbadawi
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction above.
18 + 25 O2 --> 16 CO2 + 18 H2O
89.6 g
17.3 g
34.5 g
46.2 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction...
Questions


Social Studies, 07.08.2019 00:10

Social Studies, 07.08.2019 00:10


Mathematics, 07.08.2019 00:10




Mathematics, 07.08.2019 00:10

Mathematics, 07.08.2019 00:10

Mathematics, 07.08.2019 00:10

History, 07.08.2019 00:10


Mathematics, 07.08.2019 00:10


Mathematics, 07.08.2019 00:10


Social Studies, 07.08.2019 00:10

Mathematics, 07.08.2019 00:10

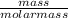
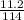 = 0.098mole
= 0.098mole 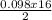 = 0.79mole of CO₂
= 0.79mole of CO₂

