

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
What is the value of delta Hrxn for this equation:
NH3(g)+2O2(g)⟶HNO3(g)+H2O(g)
Based...
Based...
Questions

Mathematics, 25.03.2020 21:01






English, 25.03.2020 21:01

Mathematics, 25.03.2020 21:01


Biology, 25.03.2020 21:01

Mathematics, 25.03.2020 21:01

History, 25.03.2020 21:01



Social Studies, 25.03.2020 21:01


Mathematics, 25.03.2020 21:01


English, 25.03.2020 21:01

Mathematics, 25.03.2020 21:01

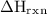 of the reaction can be 179 kJ/mol.
of the reaction can be 179 kJ/mol. 390
390 


