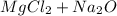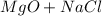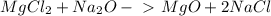For questions #14 – 17, write an equation for the reaction of magnesium chloride and sodium oxide to produce magnesium oxide with sodium chloride.
14. show the formulas of the reactants.
15. show the formulas of the products.
16. write the balanced the equation for this reaction.
17. what type of chemical reaction is this? how do you know?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

You know the right answer?
For questions #14 – 17, write an equation for the reaction of magnesium chloride and sodium oxide to...
Questions





Mathematics, 17.07.2020 23:01

Biology, 17.07.2020 23:01


English, 18.07.2020 01:01



Mathematics, 18.07.2020 01:01





Mathematics, 18.07.2020 01:01