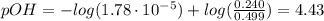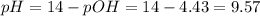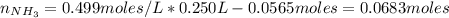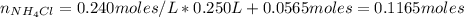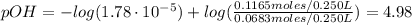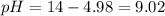Chemistry, 18.07.2020 01:01 DESIRE44030
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchloric acid are added to 250 mL of this buffer, what is the pH of the resulting solution? (Assume that the volume does not change upon adding perchloric acid.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchlo...
Questions




Geography, 29.10.2020 19:20



Mathematics, 29.10.2020 19:20

Chemistry, 29.10.2020 19:20

Mathematics, 29.10.2020 19:20


Mathematics, 29.10.2020 19:20






Mathematics, 29.10.2020 19:20


Mathematics, 29.10.2020 19:20

![pOH = pKb + log(\frac{[NH_{4}Cl]}{[NH_{3}]})](/tpl/images/0708/9694/eb1d2.png)