
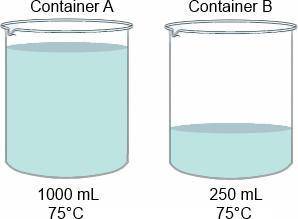
How will the temperatures of the water in the containers compare if an equal amount of heat is absorbed by each container of water without boiling?
A. Both water temperatures will increase, but container B's will increase more.
B. Both water temperatures will decrease, but container B's will decrease more.
C. Both water temperatures will increase by the same amount.
D. Both water temperatures will increase, but container A's will increase more.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
How will the temperatures of the water in the containers compare if an equal amount of heat is absor...
Questions


English, 29.01.2020 17:52


Advanced Placement (AP), 29.01.2020 17:52


Mathematics, 29.01.2020 17:52

English, 29.01.2020 17:52


History, 29.01.2020 17:52


Mathematics, 29.01.2020 17:52



Chemistry, 29.01.2020 17:52


English, 29.01.2020 17:52

Health, 29.01.2020 17:52

History, 29.01.2020 17:52




