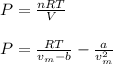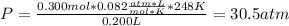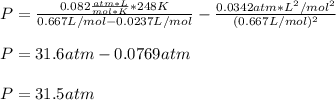Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1
You know the right answer?
For a 0.300 mol sample of helium gas in a 0.200 L container at 248K, will the pressure be greater if...
Questions

Mathematics, 11.03.2021 22:10



Mathematics, 11.03.2021 22:10


Mathematics, 11.03.2021 22:10

History, 11.03.2021 22:10

Biology, 11.03.2021 22:10




Mathematics, 11.03.2021 22:10


Mathematics, 11.03.2021 22:10

Arts, 11.03.2021 22:10

English, 11.03.2021 22:10

Mathematics, 11.03.2021 22:10