
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached below)
response, do the following:
• Label all parts (1–9), including the solutions in each beaker and the connecting tube.
• Label which cell is the cathode and which cell is the anode. Include the charge on each strip.
• Show, or describe in detail, the flow of electrons.
• Describe what type of electrochemical cell is pictured. Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.
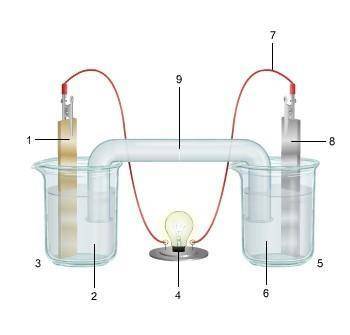

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1
You know the right answer?
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached...
Questions





English, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00






Social Studies, 22.10.2019 18:00




Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00


Mathematics, 22.10.2019 18:00




