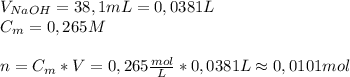Chemistry, 29.08.2019 10:10 JFrocks2480
A2.10g of unknown monoprotic acid is titrated with 38.10 ml of .265m naoh. calculate the molar mass of the ac? i already calculated that there are .0101 moles of naoh so that i have .0101 mol of the unknown acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
A2.10g of unknown monoprotic acid is titrated with 38.10 ml of .265m naoh. calculate the molar mass...
Questions





Social Studies, 23.04.2021 08:00

Arts, 23.04.2021 08:00

Chemistry, 23.04.2021 08:00




Advanced Placement (AP), 23.04.2021 08:00

Biology, 23.04.2021 08:00





English, 23.04.2021 08:00



Mathematics, 23.04.2021 08:00