
Chemistry, 23.04.2021 08:00 chiefkeef5330
Take a look at the following chemical equation. Complete the following: balance the equation, explain how the conversation of matter supports your answer, and identify the type of reaction.
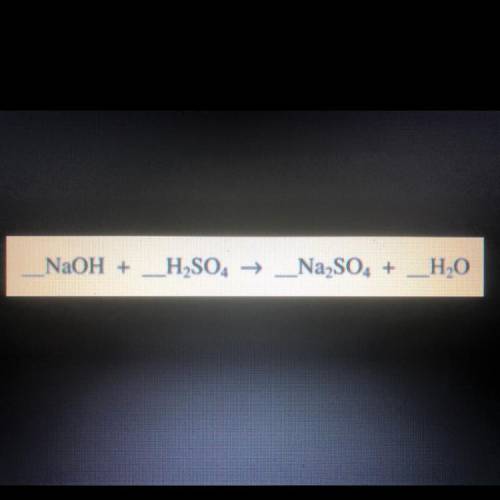

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1

Chemistry, 23.06.2019 13:00
Aecosystem is if it can continue to function over long periods of time
Answers: 1
You know the right answer?
Take a look at the following chemical equation. Complete the following: balance the equation, explai...
Questions


History, 01.12.2020 08:40



English, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40




Mathematics, 01.12.2020 08:40


Social Studies, 01.12.2020 08:40

English, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40

English, 01.12.2020 08:40

Biology, 01.12.2020 08:40

Computers and Technology, 01.12.2020 08:40



