
Can someone good in chemistry help me with these questions?
1. Calculate the rate of diffusion of NH3 gas from the data you have for distance travelled and time.
2. Calculate the rate of diffusion of HCl gas from the data you have for distance travelled and time.
3. Calculate the ratio of rate of diffusion of NH3/rate of diffusion of HCl (experimental value)
4. Calculate the ratio using grahams law of diffusion (theoretical value) rateNH3/rateHCl using Grahams law
5. Compare the two values by calculating the %deviation
Discuss these differences in the discussion part as well.
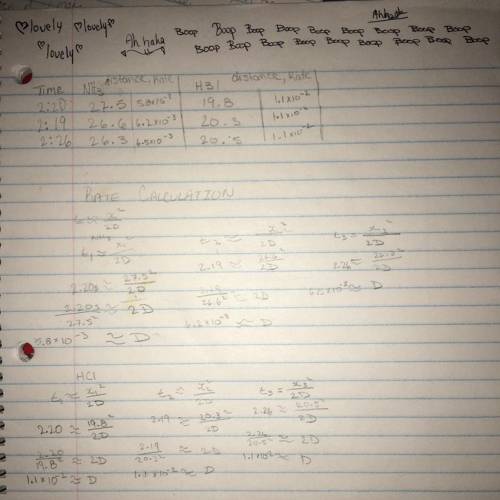

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
Can someone good in chemistry help me with these questions?
1. Calculate the rate of diffusion of N...
Questions







Biology, 18.11.2019 20:31


Chemistry, 18.11.2019 20:31




Mathematics, 18.11.2019 20:31

Chemistry, 18.11.2019 20:31





English, 18.11.2019 20:31



