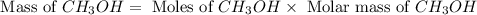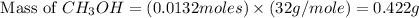Chemistry, 18.11.2019 20:31 PlaneGamer5678
Carbon monoxide gas reacts with hydrogen gas to form methanol. co(g)+2h2(g)? ch3oh(g)a 1.30l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 387mmhg .identify the limiting reactant and determine the theoretical yield of methanol in grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 09:30
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol. co(g)+2h2(g)? ch3oh(g)a 1.30l reactio...
Questions

Mathematics, 19.07.2019 01:30



Social Studies, 19.07.2019 01:30





Biology, 19.07.2019 01:40

Business, 19.07.2019 01:40

Biology, 19.07.2019 01:40

Advanced Placement (AP), 19.07.2019 01:40

Biology, 19.07.2019 01:40

Biology, 19.07.2019 01:40

Mathematics, 19.07.2019 01:40

Health, 19.07.2019 01:40




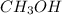 is 0.422 grams.
is 0.422 grams.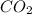 and
and 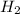 gas.
gas.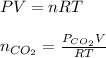
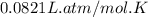
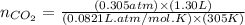
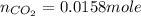
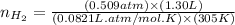
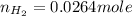
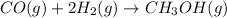
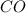
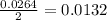 moles of
moles of