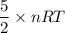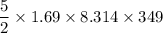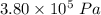A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.70 ✕ 105 Pa. The temperature of the air outside the tank is 19.0°C. The molar mass of Cl2 is 70.9 g/mol. (a) What is the volume of the tank (in m3)? m3 (b) What is the internal energy of the gas (in J)? J (c) What is the work done by the gas (in J) if the temperature and pressure inside the tank drop to 31.0°C and 3.80 ✕ 105 Pa, respectively, due to a leak? (Assume that the air outside the tank can be treated as a vacuum.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

You know the right answer?
A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.7...
Questions

English, 28.06.2019 13:30


Spanish, 28.06.2019 13:30

Biology, 28.06.2019 13:30

Social Studies, 28.06.2019 13:30



Chemistry, 28.06.2019 13:30

History, 28.06.2019 13:30

Mathematics, 28.06.2019 13:30


Mathematics, 28.06.2019 13:30

Biology, 28.06.2019 13:30




Mathematics, 28.06.2019 13:30


Social Studies, 28.06.2019 13:30

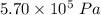
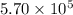
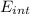 =
=