
Chemistry, 29.09.2020 23:01 COOLIOMARIS
Using the enthalpy diagram below, determine which of the following is true.
1. The reaction is exothermic
2. The energy difference between reactants and products is greater for the reverse direction than for the forward direction.
3. The combined reactants possess more energy than the combined products.
4. The activation energy in the forward direction is greater than the activation energy in the reverse direction .
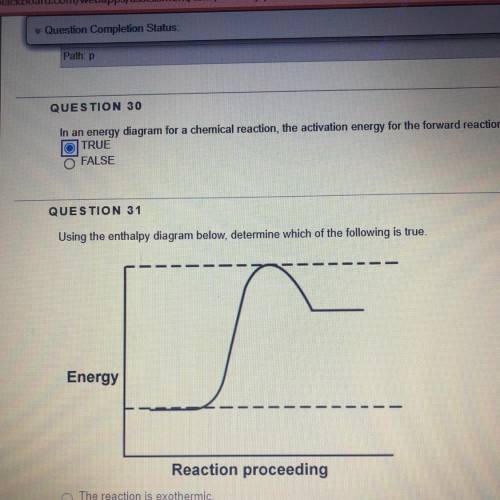

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Using the enthalpy diagram below, determine which of the following is true.
1. The reaction is exot...
Questions


Geography, 14.11.2019 07:31








Mathematics, 14.11.2019 07:31






History, 14.11.2019 07:31

Social Studies, 14.11.2019 07:31

History, 14.11.2019 07:31


Social Studies, 14.11.2019 07:31



