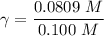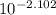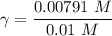Chemistry, 20.09.2020 18:01 jamesgraham577
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information, calculate the activity coefficient of H+.B. The measured pH of a solution of 0.010 HCl and 0.090 KCl at 25 degree Celsius is 2.102. Calculate the activity coefficient of H+ in this solution. C. Why does the pH change in part B relative to that in part A?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

You know the right answer?
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information,...
Questions

Mathematics, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01

English, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01


Social Studies, 25.09.2020 14:01

History, 25.09.2020 14:01

English, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01


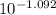
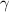 × C
× C![\gamma = \dfrac{[a]}{C}](/tpl/images/0773/2720/33e10.png)