
Chemistry, 29.08.2020 07:01 Jackiebear4593
Which of the following represents an electron configuration that corresponds to the valence electrons of an element for which there is an especially large jump between the second and third ionization energies?
A. 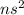
B. 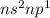
C. 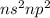
D. 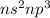

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Which of the following represents an electron configuration that corresponds to the valence electron...
Questions

Biology, 02.12.2021 20:00

Mathematics, 02.12.2021 20:00

Social Studies, 02.12.2021 20:00




Computers and Technology, 02.12.2021 20:00


Mathematics, 02.12.2021 20:00


Biology, 02.12.2021 20:00

French, 02.12.2021 20:00


Social Studies, 02.12.2021 20:00

English, 02.12.2021 20:00

Mathematics, 02.12.2021 20:00

Computers and Technology, 02.12.2021 20:00


Computers and Technology, 02.12.2021 20:00

Computers and Technology, 02.12.2021 20:00



