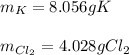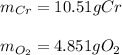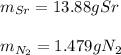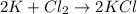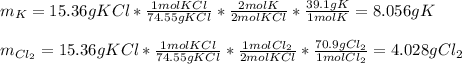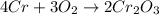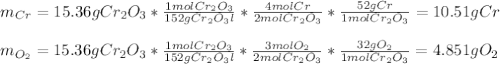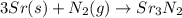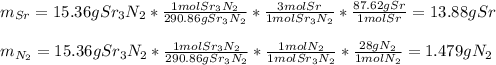Chemistry, 19.08.2020 05:01 momoney5746
For each of the following reactions calculate the mass (in grams) of both the reactants that are required to form 15.39g of the following products.
a. 2K(s) + Cl2(g) → 2Cl(aq)
b. 4Cr(s) + 302(g) → 2Cr2O3(s)
c. 35r(s) + N2(g) → SraNa(s)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1


Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
For each of the following reactions calculate the mass (in grams) of both the reactants that are req...
Questions

Mathematics, 07.12.2020 01:50


Mathematics, 07.12.2020 01:50


Social Studies, 07.12.2020 01:50


Biology, 07.12.2020 01:50

Mathematics, 07.12.2020 01:50

Business, 07.12.2020 01:50

Mathematics, 07.12.2020 01:50


Geography, 07.12.2020 01:50

Mathematics, 07.12.2020 01:50

Health, 07.12.2020 01:50

Mathematics, 07.12.2020 01:50

Law, 07.12.2020 01:50

Mathematics, 07.12.2020 01:50