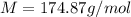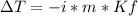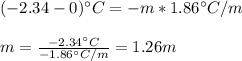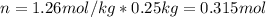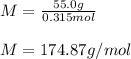Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
A solution that contains 55.0 g of ascorbic acid (Vitamin C) in 250. g of water freezes at –2.34°C....
Questions






Mathematics, 17.09.2019 22:10




Health, 17.09.2019 22:10


Mathematics, 17.09.2019 22:10


Biology, 17.09.2019 22:10

History, 17.09.2019 22:10


History, 17.09.2019 22:10