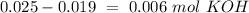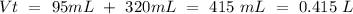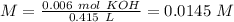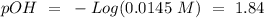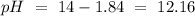Chemistry, 16.07.2020 04:01 myalee1419
Determine the pH of a solution created by mixing 95.0 mL of 0.200 M nitric acid, HNO3, with 320.0 mL of 0.078 M potassium hydroxide, KOH.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1


Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
Determine the pH of a solution created by mixing 95.0 mL of 0.200 M nitric acid, HNO3, with 320.0 mL...
Questions


Social Studies, 26.04.2021 21:10




Mathematics, 26.04.2021 21:10

Mathematics, 26.04.2021 21:10


Mathematics, 26.04.2021 21:10

Mathematics, 26.04.2021 21:10


Physics, 26.04.2021 21:10

Mathematics, 26.04.2021 21:10

Advanced Placement (AP), 26.04.2021 21:10


Mathematics, 26.04.2021 21:10

Mathematics, 26.04.2021 21:10

Mathematics, 26.04.2021 21:10


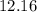
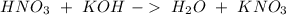
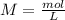 (this means that we have to find the Litters dividing by 1000)
(this means that we have to find the Litters dividing by 1000)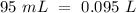
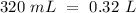
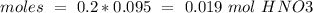
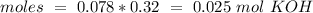
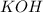 reacts with 1 mol of
reacts with 1 mol of 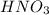 ). So, if we have
). So, if we have 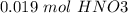 we will need
we will need 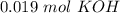 . So, we have to calculate the amount of KOH that remains in the solution, so:
. So, we have to calculate the amount of KOH that remains in the solution, so: