
Chemistry, 09.07.2020 01:01 southerntouch103
For the galvanic (voltaic) cell Fe(s) + Mn2+(aq) → Fe2+(aq) + Mn(s) (E°= 0.77 V at 25°C), what is [Fe2+] if [Mn2+] = 0.040 M and E = 0.78 V? Assume T is 298 K

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
You know the right answer?
For the galvanic (voltaic) cell Fe(s) + Mn2+(aq) → Fe2+(aq) + Mn(s) (E°= 0.77 V at 25°C), what is [F...
Questions




















![\rm E_c_e_l_l\;=\;E_0\;-\;\dfrac{0.059}{n}\;\times\;log\;\dfrac{[Fe^+]}{[Mn^2^+]}](/tpl/images/0703/3772/e799c.png)
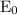 = initial voltage = 0.77 V
= initial voltage = 0.77 V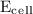 = cell voltage = 0.78 V
= cell voltage = 0.78 V![\rm \dfrac{0.059}{2}\;\times\;log\;\dfrac{[Fe^2^+]}{[0.040]}](/tpl/images/0703/3772/52542.png)
![\rm \times\;log\;\dfrac{[Fe^2^+]}{[0.040]}](/tpl/images/0703/3772/78e9d.png)
![\rm log\;\dfrac{[Fe^2^+]}{[0.040]}](/tpl/images/0703/3772/ac39f.png)
![\rm \dfrac{[Fe^2^+]}{0.040}](/tpl/images/0703/3772/f95e5.png)



