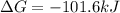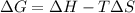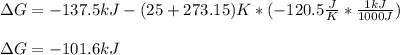Chemistry, 08.07.2020 20:01 michelle7511
Consider the following reaction: C2H4(g)+H2(g)→C2H6(g) ΔH=−137.5kJ; ΔS=−120.5J/K Calculate ΔG at 25 ∘C and determine whether the reaction is spontaneous. Express the free energy change in joules to four significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
Consider the following reaction: C2H4(g)+H2(g)→C2H6(g) ΔH=−137.5kJ; ΔS=−120.5J/K Calculate ΔG at 25...
Questions

Health, 27.08.2019 16:30


Mathematics, 27.08.2019 16:30



Health, 27.08.2019 16:30

English, 27.08.2019 16:30


Mathematics, 27.08.2019 16:30


History, 27.08.2019 16:30


Biology, 27.08.2019 16:30



Mathematics, 27.08.2019 16:30

English, 27.08.2019 16:30

Mathematics, 27.08.2019 16:30

Mathematics, 27.08.2019 16:30